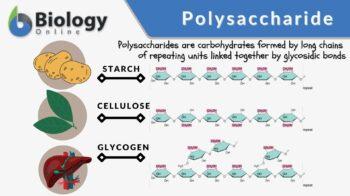
Polysaccharide
n., plural: polysaccharides
[pɒlɪˈsækəɹaɪd]
Definition: Any from the group of polymeric carbohydrates formed by long chains of repeating units linked together by glycosidic bonds
Table of Contents
Polysaccharide Definition
Biology Definition: A polysaccharide is a carbohydrate formed by long chains of repeating units linked together by glycosidic bonds. The term polysaccharide etymologically means multi saccharides. A saccharide refers to the unit structure of carbohydrates. Thus, a polysaccharide is a carbohydrate comprised of many saccharides, particularly, more than ten (mono)saccharide units. Etymology: Ancient Greek πολύς (polús, meaning “many) + saccharide. Synonyms: polysaccharose. Compare oligosaccharide. See Also: polymer, cellulose, glycogen.
Carbohydrates are organic compounds comprised of carbon, hydrogen, and oxygen, usually in the ratio of 1:2:1. They are one of the major classes of biomolecules. They are an important source of energy. They also serve as structural components. As a nutrient, they can be classified into two major groups: simple carbohydrates and complex carbohydrates.
Simple carbohydrates, sometimes referred to as, simply, sugars, are those that are readily digested and serve as a rapid source of energy. Complex carbohydrates (such as cellulose, starch, chitin, and glycogen) are those that need more time to be digested and metabolized. They often are high in fiber and unlike simple carbohydrates, they are less likely to cause spikes in blood sugar levels.
Properties of Polysaccharides
Polysaccharides are characterized by the following chemical properties:
- Not sweet in taste
- Many of which are insoluble in water
- Do not form crystals when desiccated
- Compact and not osmotically active inside the cells
- Can be extracted to form a white powder
- General chemical formula of Cx(H2O)y
Polysaccharides consist of hydrogen, carbon, and oxygen, just as the other forms of carbohydrates. The ratio of hydrogen atoms to oxygen atoms is often 2:1, which is why they are also described as hydrates of carbon. The general chemical formula of polysaccharides is (C6H10O5)n. Because of the presence of carbon and C-C and C-H covalent bonds, they are considered organic compounds similar to other carbohydrates.
Polysaccharides differ from oligosaccharides and disaccharides based on how many monosaccharide units are present. Disaccharides are made up of only two monosaccharides. Oligosaccharides have more than two monosaccharides. The term oligosaccharide is commonly used to describe relatively shorter chains than polysaccharides. Polysaccharides are a type of biological macromolecule comprised of multiple monosaccharide units.
There are diverse forms of polysaccharides. Their structure ranges from simple linear to more complex, highly branched forms. Many of them are heterogenous. Depending on their composition, they may be amorphous or water-insoluble.
Dehydration Synthesis
The chemical process of joining monosaccharide units is referred to as dehydration synthesis since it results in the release of water as a byproduct. One way of synthesizing a polysaccharide is through a condensation reaction as it involves the joining of sub-units to form a rather condensed compound with the concomitant release or loss of water.
Hydrolysis
Hydrolysis is the process of converting polysaccharides into simple monosaccharide components. While condensation reaction involves the elimination of water, hydrolysis utilizes water molecules. The process of converting polysaccharides into monosaccharides, in particular, is called saccharification.
In humans, carbohydrates (apart from monosaccharides) are digested through a series of enzymatic reactions. These enzymes are salivary amylase, pancreatic amylase, and maltase. Salivary amylase acts on the starch and breaks it down to maltose. The next site of carbohydrate digestion will be the small intestine. The stomach is not involved in the digestion of carbohydrates because gastric juice inhibits the activity of salivary amylase. Thus, the next phase of carbohydrate digestion will be the small intestine.
When the partially-digested carbohydrates reach the small intestine, the pancreas secretes pancreatic juices that include the pancreatic amylase. This enzyme acts on the partially-digested carbohydrates by breaking them down into simple sugars. The brush border of the small intestine releases digestive enzymes such as isomaltase, maltase, sucrase, and lactase.
Isomaltase digests polysaccharides at the alpha 1-6 linkages, and convert alpha-limit dextrin to maltose. Maltase breaks down maltose (a disaccharide) into two glucose units. Sucrase and lactase digest sucrose and lactose, respectively, into monosaccharide constituents. The epithelial cells at the brush border of the small intestine absorb monosaccharides. Glucose and galactose are taken inside the intestinal cell (enterocyte) via active transport using glucose transporters (GluT). Fructose is also taken up using GluT but the mode of transport is not yet clear (whether it is by active or passive transport). The enterocytes release the monosaccharides into the capillaries via passive transport (particularly, by facilitated diffusion). The simple sugars are then transported to the cells of other tissues, especially to the liver, from the bloodstream. Glucose in the blood may be utilized by the body to produce ATP. Otherwise, it is transported to the liver, together with the galactose and fructose (which are largely converted into glucose), for storage as glycogen.
The remaining carbohydrates not absorbed by the small intestine enter the large intestine. The gut flora in the colon metabolizes them anaerobically (e.g. fermentation). As such, this leads to the production of gases (e.g. hydrogen, CO2, and methane) and fatty acids, such as acetate and butyrate, that are immediately metabolized by the body. The gases, in turn, are excreted via breathing them out, eructation (burping), or flatulence.
Glycogenesis
Glycogenesis is the metabolic process of producing glycogen from glucose for storage. The process occurs mainly in liver and muscle cells in response to the high glucose levels in the bloodstream. Short polymers of glucose, especially exogenous glucose, are converted into long polymers to be stored inside the cells. When the body requires metabolic energy, glycogen is broken down into glucose subunits through the process of glycogenolysis. Thus, glycogenesis is the opposite process of glycogenolysis.
Glycogenolysis
Glycogenolysis is the process of breaking down stored glycogen in the liver so that glucose may be produced for use in energy metabolism. Stored glycogen in the liver cells is broken down into glucose precursors. A single glucose molecule is cut off from the glycogen and is converted into glucose 1-phosphate, which in turn, is transformed into glucose 6-phosphate that can enter glycolysis.
Glycosylation
Similar to oligosaccharides, some polysaccharides may serve as glycans in certain glycoconjugates. However, oligosaccharides are more often the carbohydrate component than polysaccharides. Glycosylation is the process whereby a glycan is enzymatically joined to a protein, lipid, or other organic molecules. The step-by-step processes of glycosylation vary, depending on the type of glycosylation. For instance, N-linked glycosylation is when the glycan is attached to a nitrogen atom of asparagine or arginine residue of a protein. Conversely, O-linked glycosylation is a process where O-linked glycans are attached to the hydroxyl oxygen of serine, threonine, tyrosine, hydroxylysine, or hydroxyproline side chains of a protein. It may also be the process where the O-linked glycans attach to the oxygen on lipids. Other forms of glycosylation exist, such as C-linked (i.e. glycan attached to carbon), P-linked (i.e. glycan, to phosphorus), and S-linked (glycan, to sulfur).
Classification of Polysaccharides
Polysaccharides may be a homopolysaccharide or a heteropolysaccharide depending on their monosaccharide components. A homopolysaccharide (also called homoglycan) is made up of only one type of monosaccharide whereas a heteropolysaccharide (also called heteroglycan) is composed of different types of monosaccharides.
Based on their function, polysaccharides may be classified as storage or structural polysaccharides. Storage polysaccharides are those that are used for storage. For instance, plants store glucose in the form of starch. Animals store simple sugars in the form of glycogen. Structural polysaccharides are carbohydrates that have a structural role. Plants have celluloses, which are polymers of repeated glucose units that are joined by beta-linkages. Certain animals produce chitin that serves as a structural component, for example, of the exoskeleton.
Examples of Polysaccharides
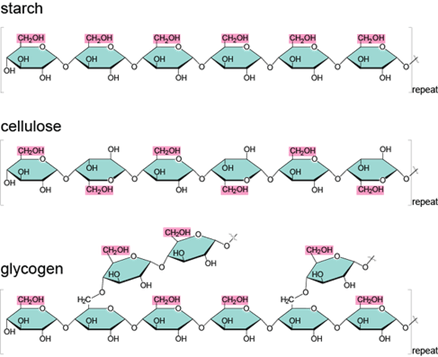
Common examples of polysaccharides are cellulose, starch, glycogen, and chitin. Cellulose is a polysaccharide consisting of a linear chain of β (1→4) linked D-glucose units: (C6H10O5) n. Starch is a polysaccharide carbohydrate (C6H10O5)n consisting of a large number of glucose monosaccharide units joined together by glycosidic bonds found especially in seeds, bulbs, and tubers. Glycogen is a branched polymer of glucose that is mainly produced in liver and muscle cells, and functions as secondary long-term energy storage in animal cells. Chitin is a polymer of nitrogen-containing polysaccharide (C8H13O5N)n rendering a tough, protective covering or structural support in certain organisms. It makes up the cell walls of fungi and exoskeleton of insects. Other examples of disaccharides are callose, chrysolaminarin, xylan, mannan, fucoidan, galactomannan, arabinoxylan.
Biological Importance
Polysaccharides, just as the other carbohydrates, are a major source of energy, and therefore are one of the main dietary components. Animals consume them to obtain monosaccharides that they can use to synthesize ATP. ATPs are chemical energy biologically synthesized through aerobic and anaerobic respiration. Glucose is the most common form of monosaccharide that the cell uses to synthesize ATP via substrate-level phosphorylation (glycolysis) and/or oxidative phosphorylation (involving redox reactions and chemiosmosis). And one of the sources of glucose is a carbohydrate-containing diet. Too much carbohydrate in the diet though could lead to health problems. Consistently high blood sugar levels could eventually lead to diabetes mellitus. The gut would also need to exert greater effort to digest them. Too much fructose, for instance, could lead to malabsorption in the small intestine. When this happens, unabsorbed fructose transported to the large intestine could be used in fermentation by the colonic flora. This could lead to gastrointestinal pain, diarrhea, flatulence, or bloating.
Plants store excess glucose in the form of starch. Thus, there are plants that are harvested to use the starch for food preparation and industrial purposes. Animals store carbohydrates in the form of glycogen so that when the body demands more glucose, glucose can be taken from this reserve through the process of glycogenolysis. Polysaccharides are also essential in living organisms as they serve as structural components of biological structures, such as cellulose and chitin. Plant cellulose is harvested for its multifarious uses in the industry.
Try to answer the quiz below to check what you have learned so far about polysaccharides.
Further Reading:
More info relating to carbohydrates and their role in our diet can be found in the biology tutorial A Balanced Diet – Carbohydrates and Fat.
© Biology Online. Content provided and moderated by Biology Online Editors
Reviewed by: Todd Smith, PhD