Table of Contents
Isotonic Definition
What does isotonic mean? The term “isotonic” is used in physiology, anatomy, and physical chemistry. See below.
Isotonic definition science/chemistry
In physical chemistry: Solutions that are categorized as having equivalent or identical osmotic pressure
Isotonic definition physiology/biology
In physiology: Again, in physiology, isotonic are the solutions that have similar solute content as that of mammalian blood
Isotonic definition anatomy
Anatomically, it refers to muscles exhibiting the same tension. Isotonic is a muscle contraction condition wherein, under same or constant tension, the length of the muscle decreases
What is isotonic? The solutions having the same tonicity are known as isotonic solutions. So, the question arises, what is the definition of tonicity? Tonicity is the estimate of relative solute concentration across a semipermeable membrane, which, therefore, is also a measure of the effective osmotic pressure gradient across a semipermeable membrane.
The amount (or extent) and direction of the movement of the solvent across a semipermeable membrane are determined by the tonicity. It is important to note that only the solute that cannot cross the semipermeable membrane is responsible for the generation of osmotic pressure gradient or tonicity. It is important to understand that iso-osmotic solutions are not always isotonic solutions and, therefore, the two terms can’t be used interchangeably.
Depending on the tonicity, solutions can be classified into three types:
- Hypertonic
- Hypotonic
- Isotonic
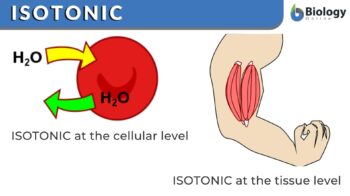
Isotonic is a descriptive word relating to isotonicity. At the cellular level, isotonicity may pertain to a property of a solution in which its solute concentration is the same as the solute concentration of another solution with which it is compared. Thus, a solution is described as isotonic when the other solution being compared with having the same (or equal) osmotic pressure and same water potential since the two solutions have an equal concentration of water molecules. It may also pertain to a condition or property of a solution that has the same tonicity as the other solution with which it is compared. For example, blood serum is isotonic to a physiologic salt solution. Solutions that have the same tonicity will result in no net flow of water across the cell membrane. Etymology: from iso– + Greek tonos (“tension”) + -ic. Compare hypotonic, hypertonic.
Hypertonic Solution vs Isotonic Solution
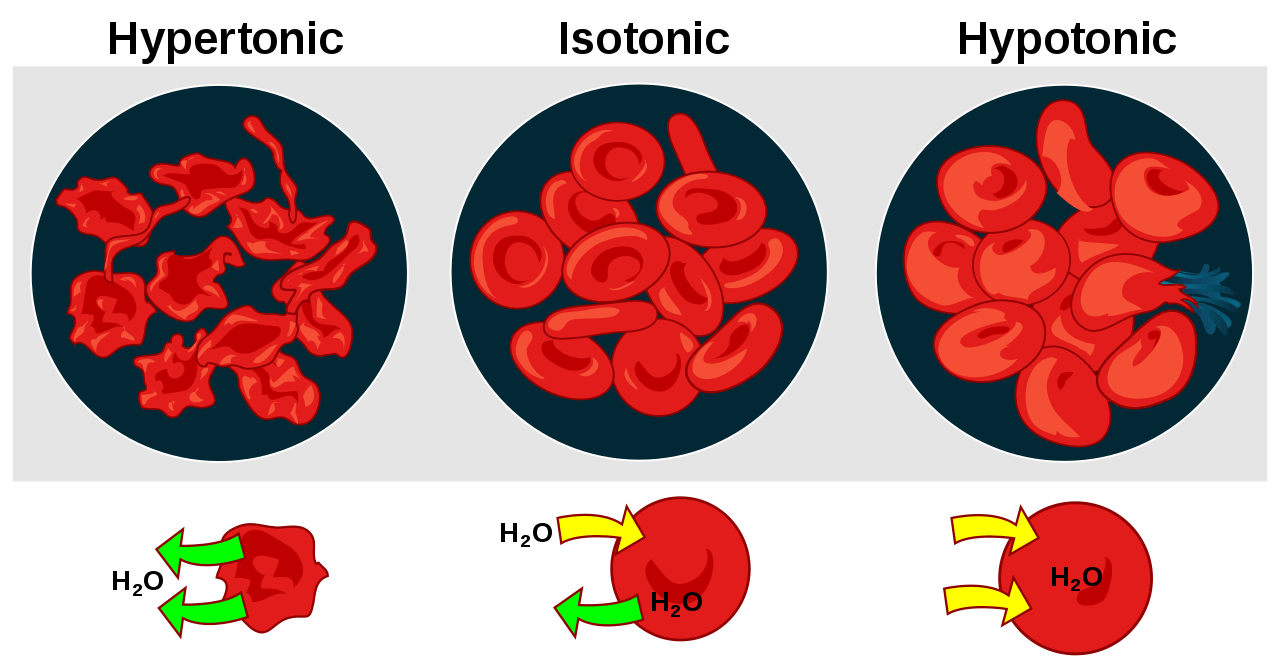
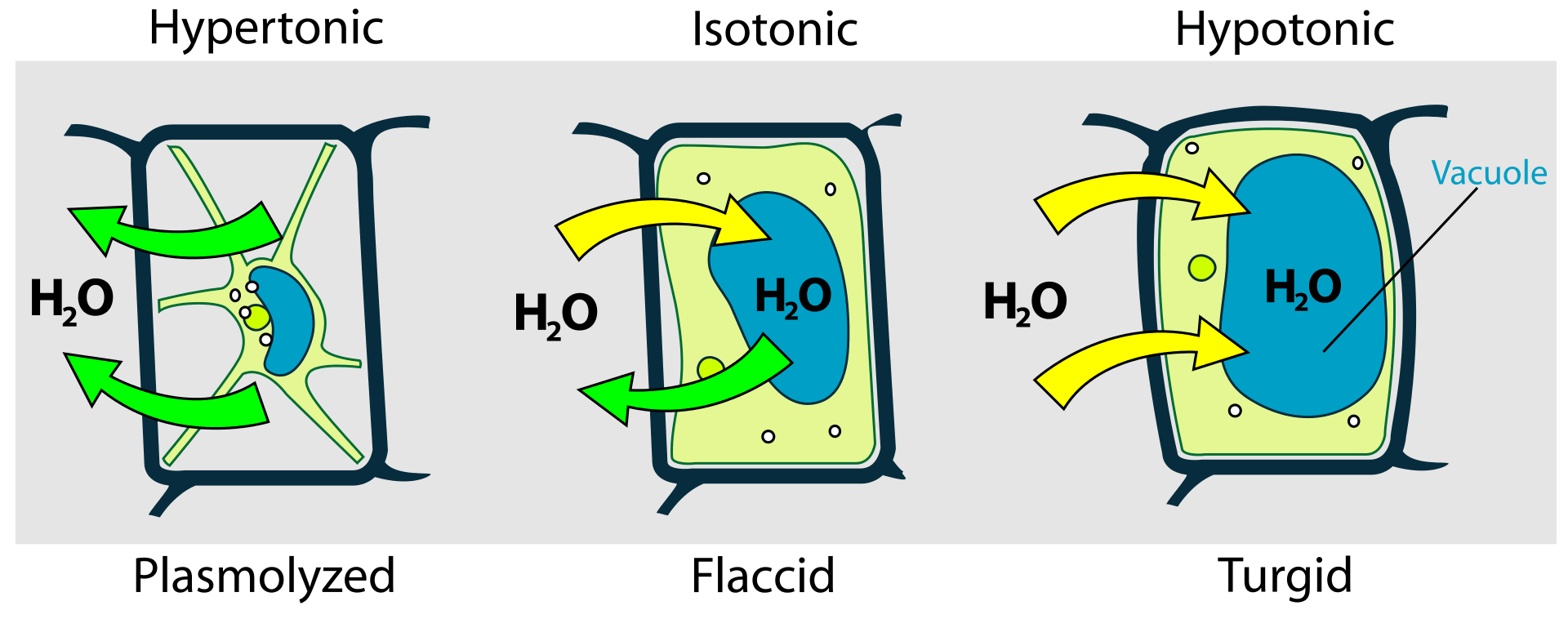
When a solution has a higher solute concentration than the solution present across the semi-permeable membrane, it is known as a hypertonic solution. As a result, when a cell is placed in a hypertonic solution, an osmotic pressure gradient forces the solvent to move out of the cell in order to attain equal solute concentration across a membrane (Figure 1). This is visualized as cell shrinkage. Thus, when a cell is placed in a hypertonic solution, it leads to shrinkage of the cell known as plasmolysis (Figure 2). In such a scenario, the cell membrane acts as a semipermeable membrane and the solution having a higher solute concentration in comparison to the cytosolic concentration is known as hypertonic.
In animal cells, the equivalent condition of plant plasmolysis is crenation. In Figure 1, notice how the red blood cells lose water as water molecules diffuse down the concentration gradient.
Hypotonic Solution vs Isotonic Solution
When a solution has a lower solute concentration than the solution present across the semipermeable membrane, it is known as a hypotonic solution. As a result, when a cell is placed in a hypotonic solution, an osmotic pressure gradient forces the solvent to move into the cell in order to attain equal solute concentration across a membrane (Figure 1). This is visualized as cell swelling.
When a cell is placed in a solution having a lower solute concentration in comparison to cytosolic concentration, the gradient difference results in the movement of the solvent into the cell resulting in swelling and eventually, bursting or cytolysis of the cell (Figure 1). Usually, this happens in the case of animal cells, which are without any cell wall. However, in the case of plant cells that have a cell wall and a central vacuole, the rigid cell wall protects the cell from bursting and the vacuoles take up the excess water pushing up the cell membrane against the cell wall. (Figure 3) This phenomenon is known as turgor pressure.
What is an Isotonic Solution?
An isotonic solution is one wherein the solute concentration across the semipermeable membrane is the same resulting in an equilibrium state. Thus, in the case of isotonic solutions due to the absence of a concentration gradient, there is no net movement of the solvent molecules across a semipermeable membrane (Figure 1). However, it does not mean that there is no movement of the solvent across the membrane. In reality, only the rate of solvent movement across the membrane is the same which results in a net-zero movement. Thus, there is neither gain nor loss of the solvent molecules across the membrane (Figure 1 & 3).
Normal saline solution (9 gm/l of sodium chloride solution) is iso-osmolar and isotonic to the blood plasma. So, what is a saline solution? Saline solution is an aqueous solution of sodium chloride, wherein 9 grams of sodium chloride is dissolved in 1 liter of the water i.e., 0.9% w/v. Due to similar sodium concentrations in blood, saline is also known as a physiological solution.
Due to isotonicity, saline solution is widely used in medicine both topically as well as parenterally (i.e., injecting directly into the bloodstream), like in cleaning wounds, sinus, fluid replacement therapy, hydration maintenance, etc. The red blood cells retain their shape. They do not shrink or swell in an isotonic saline solution, i.e., 0.9% w/v solution of sodium chloride (Figure 1).
Now, let’s suppose RBCs are placed in a solution with a sodium chloride concentration of 2% w/v. The sodium chloride concentration of 2% w/v is greater than the RBC’s sodium content and hence the solution is hypertonic. This will result in shrinkage of the RBCs known as crenation (Figure 1).
In another situation, let’s suppose RBCs are placed in a solution with a sodium chloride concentration of 0.1 % w/v. The sodium chloride concentration of 0.1% w/v is less than the RBC’s sodium content and hence hypotonic saline. This will result in swelling and eventually bursting of the RBCs which will result in the release of hemoglobin. This is known as hemolysis (Figure 1), which is a very dangerous situation.
Thus, in medicine, it is critical to administer only the isotonic saline solution intravenously, i.e., directly into the bloodstream. A hyper- or hypo-tonic solution can’t be administered via the intravenous route.
Interestingly, subcutaneous and intramuscular injection formulation can be non-isotonic, as blood cells don’t come directly in contact with the injectable formulation. However, ophthalmic solutions should be isotonic in order to avoid ophthalmic irritation and pain.
The lacrimal fluid is also isotonic with normal saline. A hypotonic solution can result in the passage of fluid into the ocular tissues causing congestion whereas a hypertonic solution will result in the extrusion of fluid from the tissue. Still, the human eye can tolerate 0.6% to 1.8% w/v.
What are examples of isotonic? Some of the examples of the isotonic solutions are as follows: normal saline, phosphate buffer saline, Lactated Ringer’s solution, Oral rehydration solutions (ORS), and Hartmann’s solution
Medical use of Isotonic solutions
- Oral Rehydration Therapy uses the isotonic solution for electrolyte supplementation and maintenance of hydration in cases like enteritis, diarrhea, etc.
- Saline treatment for treating extreme dehydration and hypernatremia (a condition wherein the serum concentration of sodium is increased).
- Saline solution is used as a vehicle for parenteral, especially intravenous, administration of drugs as saline solution and blood plasma are isotonic.
- Lactated Ringer’s solution and Hartmann’s solution are isotonic with blood plasma and used for the treatment of hypovolemia (blood volume is reduced may be due to injury or any other reason) and acidosis (blood acidity increases).
- Saline solution is used for treating rhinosinusitis.
- Saline solution is used as a vehicle for drugs that are to be administered via nebulization.
- For ophthalmic disorders
Non-Medical use of isotonic solutions
- Sports drinks for providing hydration and electrolyte supplementation
- Phosphate buffer saline is also used as a vehicle for maintaining cell cultures during experimentation
Measurement of tonicity
The tonicity of a solution can be measured by two methods:
- Hemolytic methods
This method is based on the principle of change in appearance (size and shape) of the RBCs when suspended in the test solution. The packed cell volume (PCV) of the RBCs in the test solution is compared with the reference solution and categorized as isotonic (PCVtest=PCVref) /hypertonic (PCVtest<PCVref) /hypotonic (PCVtest>PCVref). - Colligative methods
In this method, tonicity is measured using colligative properties like-
o Molecular concentration
o Freezing point data
o Sodium chloride equivalent value
o White-Vincent method
Isotonic Muscle Contraction
Isotonic contraction definition: In physiology, when the muscles change in length of the muscles resulting in a movement without a change in the muscle tension then this movement of the muscle is known as isotonic muscle contraction (Isotonic meaning-‘iso’ means same; ‘tone’ means tension). On the other hand, muscles can cause a change in the muscle tension without a change in the muscle dimension or movement via isometric muscle contractions (Isometric meaning-‘iso’ means same; ‘metric’ means length). These contractions are commonly seen in the muscles responsible for grip in the hand and forearm.
Isotonic muscle contraction can be further subdivided into:
- Concentric contractions
During this contraction, the muscles shorten to generate force to overcome resistance. For eg: Weight lifting towards the shoulders involves concentric muscle contractions. - Eccentric contractions
During this contraction, the muscles increase in length to generate force to overcome resistance. These contractions can be voluntary as well as non-voluntary.
Isotonic exercise definition
Exercises that involve a lifting phase and a lowering phase is considered to be isotonic exercises, like bicep curls and push-ups. Bicep curls involve raising the arm and lowering it while push-up exercise involves raising the body and lowering it in a plank position. Here, it is important to understand that isotonic muscles will exhibit the same muscle tone. Hence, isotonic exercise may not result from isotonic muscles. For example, in bicep curls, a person may exert more unequal focus on the right or left biceps thus resulting in non-isotonic muscles. On the contrary, in the push-ups both the sides of the body work equally, and hence they result from isotonic muscles.

Try to answer the quiz below to check what you have learned so far about isotonic.
References
- Mathias, R. T., & Wang, H. (2005). Local osmosis and isotonic transport. The Journal of membrane biology, 208(1), 39–53. https://doi.org/10.1007/s00232-005-0817-9.
- Fishman, R. A. (1953). Effects of isotonic intravenous solutions on normal and increased intracranial pressure. AMA Arch NeurPsych. 70(3):350–360. doi:10.1001/archneurpsyc.1953.02320330075007
- Baranowski, P., Karolewicz, B., Gajda, M., & Pluta, J. (2014). Ophthalmic drug dosage forms: characterization and research methods. The Scientific World Journal, 2014, 861904. https://doi.org/10.1155/2014/861904.
- Sheldon F. G. (1978). Isosmotic and Isotonic Are Not the Same. The American Biology Teacher 40 (5): 321–323. doi: https://doi.org/10.2307/4446258.
- Diamond J. M. (1964). THE MECHANISM OF ISOTONIC WATER TRANSPORT. The Journal of general physiology, 48(1), 15–42. https://doi.org/10.1085/jgp.48.1.15
- Alves, J. T., Troster, E. J., & Oliveira, C. A. (2011). Isotonic saline solution as maintenance intravenous fluid therapy to prevent acquired hyponatremia in hospitalized children. Jornal de pediatria, 87(6), 478–486. https://doi.org/10.2223/JPED.2133.
- Mathur, A., Duke, T., Kukuruzovic, R., & South, M. (2004). Hypotonic vs isotonic saline solutions for intravenous fluid management of acute infections. The Cochrane database of systematic reviews, 2003(2), CD004169. https://doi.org/10.1002/14651858.CD004169.pub2.
©BiologyOnline.com. Content provided and moderated by BiologyOnline Editors.